Table of contents
- 5 pillars of a healthy gut microbiome
- Pillar 1: The right diet for the gut microbiome
- Pillar 2: The right way to deal with stress
- Pillar 3: Sufficient exercise for a healthy gut microbiome
- Pillar 4: Sufficient restful sleep for our gut bacteria
- Pillar 5: Avoiding toxins like alcohol
- Get in control of your gut health
Most of us are familiar with digestive problems. Whether it’s diarrhoea, constipation, bloating or abdominal pain, these symptoms are uncomfortable and can significantly affect daily life.
The underlying cause is often an imbalance in the bacteria that make up our gut flora – also known as the gut microbiome. What many people don’t realise is that an imbalance in gut bacteria can show up in far more ways than digestive discomfort alone.
Conditions such as irritable bowel syndrome, leaky gut, weight-related issues, and problems affecting the skin, liver, thyroid, joints, inflammatory processes, mental wellbeing and insulin regulation have all been linked to the state of the gut. Many other health conditions may also be influenced by the health of our microbiome.
5 pillars of a healthy gut microbiome
However, the gut microbiome is not only responsible for ailments, but is the prerequisite for our health and general well-being. If our gut is doing well, we usually feel healthier and more vital. Although the basic microbiome is determined by our genetics, we can have a positive effect on our gut bacteria through diet and lifestyle and promote a healthy balance. At myBioma we summarise 5 pillars of a healthy gut microbiome: Nutrition, stress reduction or the right way to deal with stress, sufficient exercise and sleep, and avoiding toxins such as alcohol. In the following, we will go into detail about the 5 pillars so that you can make your diet and lifestyle microbiome-friendly.
Pillar 1: The right diet for the gut microbiome
A gut-friendly diet affects your gut like proper care affects a beautiful garden. With food, we can feed the right bacteria to multiply (the beautiful flowers in the garden) and avoid the growth of unwanted bacteria (like the weeds in the garden). Our gut bacteria particularly like vegetables, fruit, whole grains, probiotic and fermented foods, such as sauerkraut or yoghurt, and tend to react in a stressed way to saturated fats, processed foods and industrial sugars.

Figure 1: Illustration of diversity by consumption of fermented vegetables and sauerkraut (after Thiene et al., 2022)
Changing your diet can be challenging, so it makes sense to take it one step at a time rather than changing everything overnight. The first step in the right direction can be fermented foods. A recent study by Thriene et al. looked at the effects of fermented vegetables and sauerkraut on alpha diversity. Diversity describes the diversity of bacteria in the microbiome. The more different bacteria live in the gut and the better distributed their ratio, the higher the diversity. Low diversity is associated with many diseases such as diabetes, obesity, thyroid disease, depression, etc. The alpha diversity describes the composition of the gut bacteria in an individual and is described with the Shannon index. Beta diversity compares the diversity of at least two different individuals. This is used when comparing two population groups.
The study participants of Thriene et al. consumed 150 g of sauerkraut or a variety of six different commercially available fermented vegetables daily for a fortnight. Figure 1 shows how the alpha diversity increased after the diet with sauerkraut (light blue) and fermented vegetables (yellow). (1) So nothing stands in the way of consuming fermented vegetables, because they influence the bioactive substances and beneficial gut bacteria. If you are now in the mood for fermented foods, we recommend the myBioma cookbook: Microbiome food - recipes for your gut bacteria .
Fermented foods are considered psychobiotics. Psychobiotics are any interventions that affect the gut-brain axis.(2) Examples would be: Prebiotics (salsify), probiotics (kefir), postbiotics (SCFA) and synbiotics (combination of prebiotics and probiotics).
The psychobiotic effect is a complex process between probiotics, prebiotics, postbiotics, gut hormones, neurotransmitters and gut bacteria. One can imagine an interaction of all participants like in a small assembly line factory: with probiotics, bifidobacteria and lactobacilli move into the gut. Prebiotics provide those bacteria with food. Short-chain fatty acids are formed through the supply of pro- and prebiotics, which in turn activates intestinal hormones. Short-chain fatty acids and gut hormones can subsequently influence the central nervous system. And then there are the neurotransmitters. Neurotransmitters are like telephones that pass information from one nerve cell to another via synapses. Those neurotransmitters are also influenced by psychobiotics, a real interplay between them. Who must not be forgotten is the vagus nerve, because it enables the connection between the gut and the brain. We often hear that stress has a negative effect on the gut, which brings us directly to the second pillar, stress.
Pillar 2: The right way to deal with stress
Stress is almost unavoidable in today’s world and is necessary in many situations to perform well. However, stress can also lead to anxiety and depression. It is not the stress itself that makes us ill, but the wrong stress management. Those who experience stress without negative emotional effects show a high resistance to stress. This is influenced by a variety of factors, including the gut microbiome. This is where the gut-brain axis comes into play. Some studies show that there is a connection between the gut-brain axis and stress resistance or stress resilience. It is thought that short-chain fatty acids (SCFA), for example, have a positive effect on mood. (3)
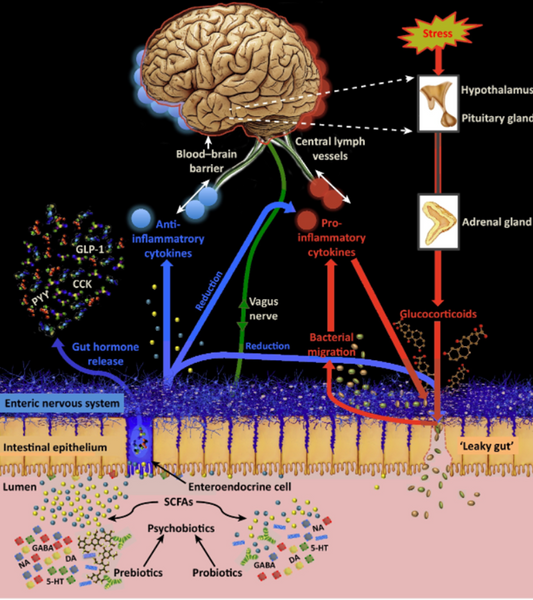
Figure 2: Illustration of the psychobiotic influence on the microbiome. influence on the microbiome (after Sarkar et al.).
In Figure 2, the red arrows show how stress affects the gut barrier and the gut-brain barrier. The barrier function is negatively influenced by stress, because glucocorticoids are emitted during stress, which in turn disrupt the Barrie. Pro-inflammatory substances subsequently promote inflammation and thus also pro-inflammatory cytokines. These have a negative influence on the intestinal barrier and the blood-brain barrier. The psychobiotic influence is shown in blue in Figure 2. Psychobiotics can reduce glucocorticoids and pro-inflammatory cytokines and help rebuild the gut barrier. They also have a positive effect on the gut-brain barrier.(2)
Indeed, research on the gut stress axis is in its infancy and still needs much in-depth research. However, one thing is certain: good stress management has a positive effect on our health and our microbiome.
Pillar 3: Sufficient exercise for a healthy gut microbiome
Exercise is good for us, that’s nothing new. However, more and more studies indicate that exercise also has an effect on alpha diversity. According to Miranda-Comas et al., short-chain fatty acids (SCFA) are formed more frequently. We remember that SCFA have an anti-inflammatory effect, they strengthen the intestinal barrier and serve as food for the intestinal epithelial cells. They have a positive effect on our immune system. (4) Learn more about this in: How are exercise and the microbiome connected?
Pillar 4: Sufficient restful sleep for our gut bacteria
A good night’s sleep makes us feel fitter and more productive. Sleep is vital for maintaining various functions in the body. Our gut bacteria are also happy to get enough sleep. (8) Unhealthy sleep patterns can lead to a decrease in diversity and negatively affect the gut-brain axis. In turn, gut bacteria influence our sleep health. (9) It’s a give and take: if we sleep enough, we promote the positive gut bacteria and they promote our sleep hygiene.
Pillar 5: Avoiding toxins like alcohol
It is no secret that excessive alcohol consumption has a negative effect on our health. Sometimes it can be one glass too many, but don’t worry, our microbiome helps us fully recover. However, the effect of excessive alcohol consumption on the microbiome should not go unnoticed. (5) The study by Leclercq et al. showed that alcohol decreased faecalibacteria, which have anti-inflammatory effects (6). In addition, another study illustrated that the integrity of the gastrointestinal barrier decreased due to the decrease in Roseburia (7). Alcohol is difficult to metabolise, can severely stress or damage the liver, and can lead to an imbalance of gut bacteria. So it’s better to skip the beer and go for water, your gut bacteria will thank you for it!
Get in control of your gut health
In summary, we would like to show you that the microbiome can be changed. It is in your hands and you can positively influence your microbiome. To find out how your own gut microbiome is doing and how it affects your health, you can test it from the comfort of your own home. With myBioma, you get a comprehensive report on your gut health and additional lifestyle and dietary suggestions to help balance your microbiome.
References
- Thriene K. et al. Effects of Fermented Vegetable Consumption on Human Gut Microbiome Diversity—A Pilot Study. Fermentation. ;8(3).(2022).
- Sarka A et al. Pychobiotucs and the Manipulation of Bacteria-Gut-Brain Signlas. Trends in Neurosciences. (2016).
- Bear T et al. The Microbiome-Gut-Brain Axis and Resilience to Developing Anxiety or Depression under Stress. Microorganisms. (2021)
- Miranda-Comas G. et al. Implications of the Gut Microbiome in Sports. Sports Health (2022).
- Day AW. et al. Gut Microbiome Dysbiosis in Alcoholism: Consequences for Health and Recovery. (2022).
- Leclercq S. et al. Alterations of kynurenine pathway in alcohol use disorder and abstinence: a link with gut microbiota, peripheral inflammation and psychological symptoms. Transl Psychiatry. ;11(1):503. (2021).
- Seo B et al. Roseburia spp. Abundance Associates with Alcohol Consumption in Humans and Its Administration Ameliorates Alcoholic Fatty Liver in Mice. Cell host & microbe. ;27(1):25-40.e6. (2020).
- Flikkema J. The Relationship Between the Gut Microbiome and Sleep Examined Through Associated Human Disease. University Honors Theses. (2022).
- Han M. et al.. The interplay between sleep and gut microbiota. Brain Research Bulletin. (2022).